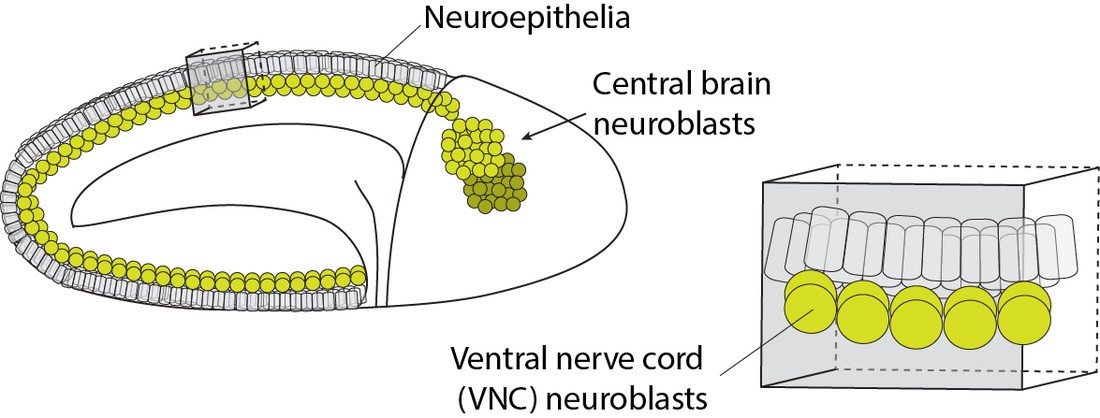
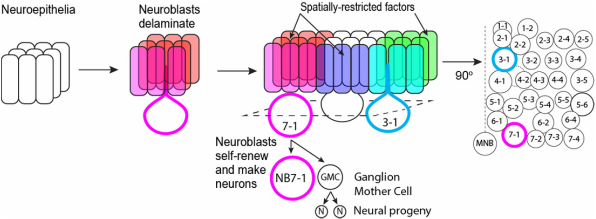
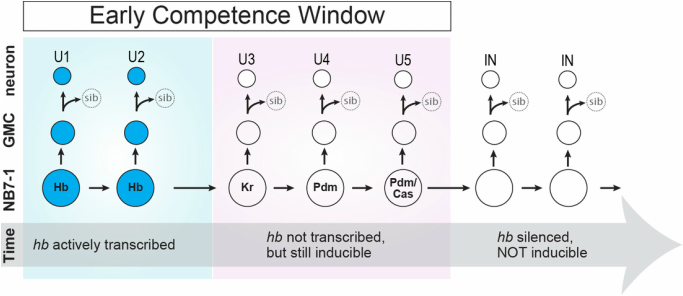
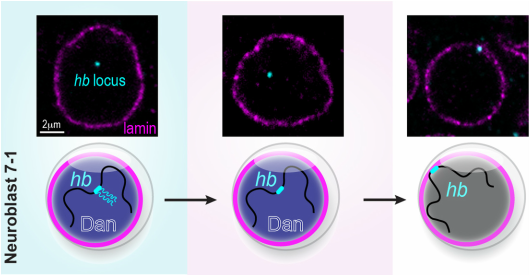
We discovered that the hb genomic locus (cyan) becomes repositioned from the interior of the NB nucleus to the periphery (nuclear lamin marked in magenta) as the NB ages over time. In NB7-1, this relocation occurs not at the end of hb transcription (division 2), but rather, at the end of the early competence window (div 5). Distal antenna (Dan, dark blue) is a nuclear factor that is transiently expressed in the NBs, and its downregulation is required to allow hb gene repositioning and the closure of the early competence window. Images modified from Kohwi et al., Cell, 2013.
Questions we are interested in addressing:
What are the mechanisms by which neuroblast competence transitions developmentally timed?
How does Dan function in nuclear architecture? What other genes are regulated in this manner?
What is the biological consequence of nuclear architecture reorganization in attaining neural diversity during brain development?